Research

Eukaryotic cells contain an endomembrane system that are comprised of morphologically and functionally distinct membrane-bound compartments (i.e., organelles). Transport of proteins and lipids between organelles is mediated by coated vesicles that are generated from one compartment, traffic to their destinations, lose their coat and fuse with another compartment. Docking and fusion of transport vesicles with the target membrane involve an initial contact mediated by Rab GTPases and tethering factors, followed by SNARE-mediated membrane fusion. Our laboratory has been interested in the SNARE-mediated fusion mechanism of the endoplasmic reticulum (ER), as well as the mechanism of the organization of the ER.
2. Studies of biogenesis and maintenance of the endoplasmic reticulum
1. Studies of syntaxin 18 (Syn18) complexes
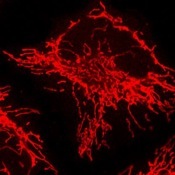


Endoplasmic reticulum (ER)
Golgi apparatus
Mitochondria
Membrane fusion occurs when one SNARE (SNAP receptor) protein on vesicles (v-SNARE) form a complex with three (in some cases two) SNARE proteins on the target membrane (t-SNAREs). The trans-SNARE complex in the fused membrane is disassembled by a chaperone-like ATPase, NSF (N-ethylmaleimide-sensitive factor), whose association with this complex is mediated by α-SNAP (soluble NSF attachment protein).
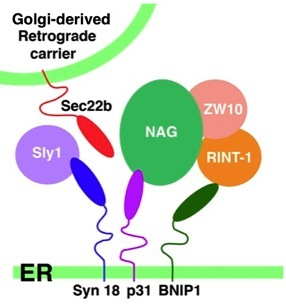
We identified Syn18 as an α-SNAP-interacting protein, and also showed that it is an ER-localized t-SNARE and makes a complex comprised of t-SNAREs, p31/Use1 and BNIP1, a v-SNARE, Sec22b, and peripheral membrane proteins, ZW10, RINT-1, NAG, and Sly1. These factors are involved not only in vesicle trafficking from the Golgi apparatus to the ER, but also in membrane fusion of the ER, the spindle or G2/M checkpoint, and apoptosis, suggesting that Syn18 complex is a key component that mediates the interplays of membrane traffic with signal transduction, cell cycle and cell survival.
More recently, we have identified a novel function of RINT-1 in the trans-Golgi network (TGN). At the TGN RINT-1 is likely associated with the Conserved Oligomeric Golgi (COG) complex, a multimeric tethering complex for fusogenic SNARE, and functions in membrane trafficking from endosomes to the TGN. Both the ZW10 complex (yeast Dsl1 complex) including RINT-1 and ZW10 and the COG complex comprise a multisubunit tethering complex family called "CATCHR" together with exocyst and GARP complexes. Our findings highlight the significance of a cooperation of the two CATCHR family complexes in the complicated endocytic trafficking pathways of mammalian cells.
The ER plays important roles in a wide variety of cellular functions including synthesis, degradation and trafficking of proteins and lipids, stress response, calcium homeostasis, and apoptosis. The ER is not only a simple continuous membrane but composed of complex subdomains and membrane contact sites with other organelles. Several lines of evidence indicate that the integrity of the ER is highly rerated to genetic disorders, especially neurodegenerative diseases, highlighting the importance of understanding the molecular mechanisms for the biogenesis and maintenance of the ER subdomains.
We have reported the functions of Syn18 and an ER-localized isoform of Syn5 in the biogenesis and maintenance of smooth ER and the interaction between the ER and microtubules, respectively. In addition, we have identified ER-localized protein Bap31 as a component of the ER quality control compartment (QCC), which is a perinuclear subdomain of the ER functioning in ER-associated protein degradation (ERAD). This protein is unique in that it cycles between the QCC and cell peripheral ER in a microtubule-dependent manner.
3. Other studies
Other projects going on in the laboratory include the understanding of the roles of small GTPases (Arf and Rab) and SNAREs in the movement and metastasis of cancer cells and Legionella pneumophila infection, and the elucidation of the molecular mechanism of the formation of vesicles that move from the TGN to the cell surface.
