Welcome to the Laboratory of Cell signaling.
Our focus is research on intracellular membrane trafficking. Our approach to these topics includes use of biochemical,
cell biological and genetic techniques.
Our laboratory consists of about 10 students and staff. You can feel at
home. You can consult senior students and staff who will give you qualified
advice.
We are actively recruiting undergraduate and graduate students to enjoy science together. The email address is shown below. We look forward to your participation.
Thank you.
Yuki Maemoto, Ph. D.
ymaemoto(a)ls.toyaku.ac.jp
Research Themes
The endoplasmic reticulum (ER) is the starting point of the secretory pathway. Once incorporated into the ER, secretory proteins are transported to the Golgi apparatus and then to the cell surface. In addition, the ER is adjacent to and/or in direct contact with mitochondria, peroxisomes, and lipid droplets, which are not involved in the secretory pathway. The ER thus plays important roles in their formation and functions. The ER is a versatile multi-domain organelle that maintains a continuous membrane structure. We are focusing on ER-mediated transport and we are studying ER functions.
Functional Analysis of Intracellular Phospholipase A1
We are focusing on the intracellular phospholipase A1 family, which is an intracellular enzyme family that converts lipids. This family consists of three members, two of which have been identified by our laboratory. Cell biological analysis has revealed roles of these enzymes in intracellular transport and in the formation of organelles. In order to address the functions of the enzymes at an organism level, we have gene-targeted the three members of this family. The resulting KO mice are now used for analysis of their physiological functions.
ER-mediated Transport Systems
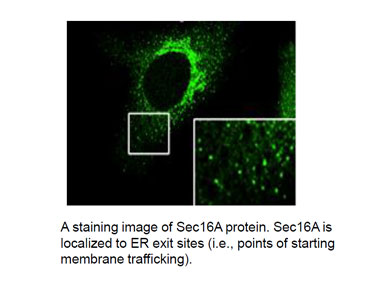
Research Content
A eukaryotic cell contains various organelles. Each organelle has distinct
morphologies and functions. The organelles are coordinated to make the
cell function as a unit. We are focusing on roles of the endoplasmic reticulum
(ER). The ER is the starting point of the secretory pathway, i.e. membrane
trafficking. In this system, secretory proteins are incorporated into a
transport vesicle, a carrier consisting of specific proteins and lipids,
and travel from the ER to the cell surface via the Golgi apparatus. Dysfunction
of the membrane trafficking is responsible for cell abnormalities, thereby
causing various diseases in organisms. We identified factors involved in
the membrane trafficking from the ER, and are now characterizing the factors
at cell and organism levels (1-3). Recently, it has been reported that
the ER is adjacent to and/or in contact with organelles other than those
involved with the secretory pathway. Examples of the organelles include
mitochondria, peroxisomes, and lipid droplets. The ER thus plays important
roles in their formation and functions. Its membrane contact sites, in
particular, have drawn attention as a novel transport system. We have reported
a factor involved in transport from the ER to peroxisomes (4-6), and are
now analyzing functions of this factor.
References
1) Shimoi et al. (2005) J. Biol. Chem. 280, 10141
2) Iinuma et al. (2007) J. Biol. Chem. 282, 17632
3) Iinuma et al. (2009) J. Cell Sci. 122, 1680
4) Yonekawa et al. (2011) PNAS 108, 12746
5) Tani et al. (2011) Cell Logist. 1, 164-167
6) Tani et al.(2013)Seikagaku 85, 30-33
Functional Analysis of Intracellular Phospholipase A1
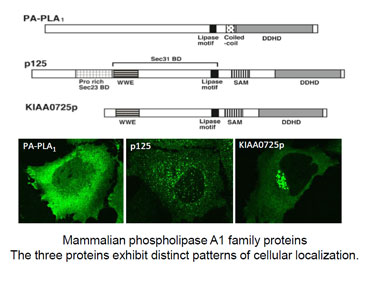
Research Content
The intracellular phospholipase A1 hydrolyzes the sn-1 ester bond of phospholipids to produce 2-acyl lysophospholipid and a free fatty acid.
This enzyme is ubiquitously expressed in eukaryotic cells and exerts effects on the membranes of various organelles.
We are analyzing, at cell and organism levels, three mammalian intracellular phospholipase A1 proteins (PA-PLA1/DDHD1, p125/Secc23IP, KIAA0725p/DDHD2) (7-9).
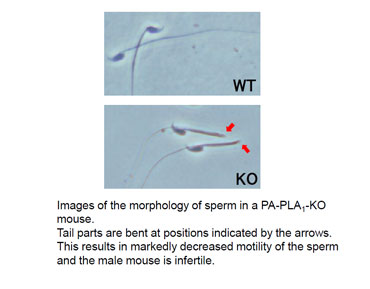
Recently, we have demonstrated that PA-PLA1/DDHD1 regulates mitochondrial dynamics and mouse sperm formation (10).
Also, a team including our group has reported a relationship between a novel mutation of KIAA0725p/DDHD2 and hereditary spastic paraplegia (HSP) (11).
Currently, we are using the KO mice to further analyze their neurological dysfunction.
References
7) Arimitsu et al. (2011) FEBS Lett. 585, 2171
8) Inoue et al. (2012) Biochim. Biophys. Acta. 1823, 930
9) Tani et al. (2012) Biomol. Concepts 3, 471
10) Baba et al. (2014) J. Biol. Chem. 289, 11497
11) Doi et al. (2014) Sci. Rep. 4, 7132
